Innovations in Cardiovascular Health:
How Academic Medicine Propelled PCSK9 From Novel Finding to Therapeutic Target
Bridgette A. Christopher, M.D., Ph.D.
Division of Cardiology, Sarah W. Stedman Nutrition and Metabolism Center and Duke Molecular Physiology Institute, Duke University School of Medicine, Durham, North Carolina
Ann Marie Navar, M.D., Ph.D.
Division of Cardiology and Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina
Introduction: The Need for New Lipid-Lowering Therapies
Atherosclerotic cardiovascular disease (ASCVD) is the No. 1 cause of death globally and affects more than 16.5 million people in the U.S.1 Improvements in short- and long-term treatments for those who develop ASCVD have decreased the mortality of myocardial infarction and stroke,2 and discoveries in primary and secondary prevention have led to reductions in the incidence of coronary heart disease over time in the U.S.3 However, despite improvements in care, adults with established cardiovascular disease remain at high risk for recurrent events.4
Basic science research spanning more than 100 years uncovered the key role of cholesterol in the development of atherosclerosis. Academic scientists first discovered cholesterol within atherosclerotic plaques in 1910.5,6 This novel discovery drove investigation into the mechanism of familial hypercholesterolemia, the association between elevated cholesterol and risk of myocardial infarction, the description of cholesterol biosynthesis and the identification of low- density lipoprotein cholesterol (LDL-C).7 Because of the rigorous work of multiple academic laboratories, the first inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG- CoA reductase), the rate-limiting step in cholesterol synthesis, was discovered in 1976.8,9,10,11 Industry took note of the potential therapeutic implications of HMG-CoA reductase inhibitors, and lovastatin became the first FDA-approved HMG-CoA reductase inhibitor in 1987 (Figure 1). Since that time, randomized trials have shown the efficacy of lipid lowering in lowering the risk of recurrent events in people with established ASCVD.12,13
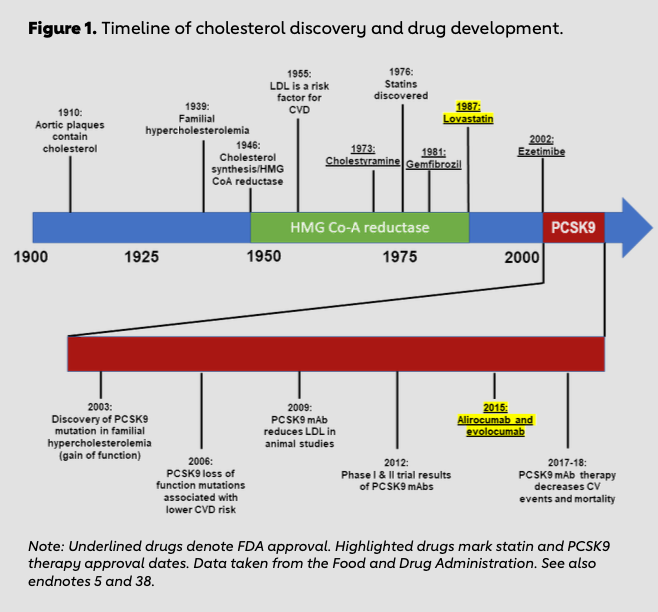
Nevertheless, not all patients’ lipid-related risk is controlled on statin therapy. Many patients are unable to take statins due to perceived side effects.14 Furthermore, many patients continue to have elevated LDL-C even on statin therapy. Therefore, additional LDL-reducing therapies with proven mortality benefit are needed to optimally treat high-risk patients with residual ASCVD risk despite statin therapy. One such class of therapy, PCSK9 inhibitors, emphasizes the success that can be achieved with rapid translation of academic discovery to pharmaceutical therapy.
Case Study: The Fast Track of PCSK9 Discovery
The story of PCSK9 inhibitor development highlights the ways in which academic and government-funded researchers play a critical role in drug discovery. One of the most influential discoveries in the treatment of ASCVD began with genetic analysis of high-ASCVD-risk families. Scientists analyzed populations to find extreme phenotypes, such as very high LDL-C, and genetic studies of these unique populations searched for novel causative genes. An academic research team in France, led by Dr. Catherine Boileau, used this method to study genetic causes of familial hypercholesterolemia (FH). Dr. Boileau heads the genetics department at Bichat University Hospital in Paris, France, and she leads a collaborative academic research team focused on genetic causes of cardiovascular diseases. Through the French Research Network for Autosomal Dominant Hypercholesterolemia, families with known hypercholesterolemia were recruited for genetic research from varying regions across France. Dr. Marianne Abifadel, a researcher in Dr. Boileau’s lab, led a study in which patients with elevated LDL-C were studied for novel genetic mutations. This study involved excluding patients with previously known FH causative mutations, including LDLR and APOB genes, and then using genetic mapping strategies to search for new mutations. Using this strategy, two new gain-of-function mutations were mapped to the PCSK9 gene.15 Research into PCSK9 exploded, with multiple groups working concurrently to better understand the role of the protein in regulation of cholesterol metabolism. Academic research teams in Norway and the U.S. described the effect of PCSK9 mutation on LDL receptors,16,17 and an academic lab at the University of Texas Southwestern Medical Center confirmed the relationship between PCSK9 mutation and plasma cholesterol.18
International Academic Researchers Contributing to Discovery
While Drs. Abifadel and Boileau et al. found gain-of-function mutations in PCSK9 in patients with elevated LDL-C levels, an academic research team in Texas focused on whether patients with very low levels of LDL-C might also harbor previously undescribed loss-of-function mutations in PCSK9. In 2005, Drs. Cohen and Helen Hobbs et al. from the University of Texas Southwestern Medical Center used the Dallas Heart Study to identify unique patient cohorts. The Dallas Heart Study began in 1999 when Drs. Ronald Victor and Hobbs from UT Southwestern sought funding from the Donald W. Reynolds Foundation to establish a population-based cohort for comprehensive cardiovascular clinical investigation.19 The Dallas Heart Study has a multiethnic population that allows for study of both social determinants and genetic mechanisms of cardiovascular disease.20 Drs. Cohen and Hobbs identified patients in the study with the lowest LDL-C levels and sequenced their PCSK9 genes. This technique identified two loss-of-function mutations present in seven patients with very low LDL-C levels.21 Drs. Cohen and Hobbs then verified that PCSK9 loss-of-function mutations were associated with a decreased risk of coronary artery disease in the Atherosclerosis Risk in Communities (ARIC) cohort.22 The ARIC study is funded through the National Heart, Lung, and Blood Institute in the National Institutes of Health, and it seeks to identify genetic and environmental determinants of atherosclerotic cardiovascular disease.23 Genetic evaluation of two separate patient populations in the DHS and ARIC validated the importance of PCSK9 in atherosclerotic risk.
Additional academic research from scientists around the globe validated the role of PCSK9 in cholesterol trafficking and demonstrated that loss-of-function mutations in PCSK9 are associated with decreased LDL and decreased coronary artery disease risk.24,25,26
The academic discoveries made by Drs. Cohen and Hobbs highlight how novel genetic discoveries can be translated into therapeutic targets. Drs. Cohen and Hobbs reached out to industry partners with their findings, with some companies willing to investigate PCSK9 and others interested in only more traditional targets, such as high-density lipoprotein cholesterol or C-reactive protein.27 When asked how she thought the PCSK9 story would change future research, Dr. Hobbs replied: “It already has had a major impact. Pharma is paying much closer attention to human genetics to both find new targets (sclerostin, Nav1.7, APOC3, ANGPTL3 …) and to determine if the effects of a drug are due to on-target or off-target effect.“28 The PCSK9 genetic discovery launched a new paradigm of how academic discoveries can lead to future therapeutics in a previously unprecedented timeline (Figure 1).
Rapid Pace of Development: The Decade between Discovery and Drug
Once PCSK9 was identified as an important modulator of plasma LDL levels and cardiovascular risk, the race to develop therapeutic strategies began. Given that loss-of-function mutations led to decreased plasma LDL-C levels and decreased coronary artery disease, experimental therapies were aimed at decreasing PCSK9 binding to LDL receptors. Decreasing PCSK9 protein levels can be accomplished by decreasing PCSK9 protein synthesis, using tools such as antisense oligonucleotides (ASO) or small interfering RNA (siRNA), or by targeting the PCSK9 protein itself with small protein inhibitors or antibodies. The first class of therapies to be evaluated in clinical trials was the monoclonal antibody inhibition of PCSK9.
In 2012, less than 10 years from the initial discovery of PCSK9, two phase 1 clinical trials published results on anti-PCSK9 monoclonal antibodies. Both Sanofi-Aventis/Regeneron29 and Amgen30,31 developed fully human monoclonal antibodies targeting PCSK9, and both phase 1 clinical trials showed significant decreases in plasma LDL-C levels without significant adverse events. In 2015, the FDA approved both alirocumab and evolocumab for the treatment of adults with heterozygous familial hypercholesterolemia or clinical ASCVD who require additional LDL- C lowering, in conjunction with diet and maximally tolerated statin therapy.32 However, the development of these FDA-approved immunotherapies has not been without controversy. In 2014, Amgen sued Sanofi-Aventis/Regeneron for patent infringement. Amgen claimed its patent encompassed an entire class of antibodies directed at PCSK9, and the Sanofi- Aventis/Regeneron product evolocumab infringed on this patent.33 An initial hearing ruled for a permanent injunction against the sale of evolocumab, but an appeal found that Amgen could not hold a patent on an entire class of antibodies.34 Sanofi-Aventis/Regeneron was able to continue the sale of evolocumab, though the lawsuit is ongoing.
Large outcomes trials have demonstrated the efficacy of alirocumab and evolocumab for reducing cardiovascular events.35,36,37 Further work in the PCSK9 space has been mixed. Bococizumab, a humanized anti-PCSK9 antibody, was withdrawn from the development pipeline due to concerns about anti-drug neutralizing antibodies.38 Work continues on alternatives to injectable antibodies for PCSK9 inhibition. A clinical trial of antisense oligonucleotides was terminated after injection-site reactions and one case of kidney injury.39 Inclisiran, an siRNA therapy, has completed phase 2 trials.40 Small protein inhibitors, including peptides and adnectins, are also in development.41 Phase 1 clinical trials of vaccine peptides are underway, with initial results demonstrating that an anti-PCSK9 vaccine can induce patient development of high-level, persistent antibodies against PCSK9.42
New Barriers to Dissemination of Innovation
The successful development of PCSK9 inhibitors has not translated into widespread use of PCSK9 inhibitor therapy to lower the risk of cardiovascular disease. Rather, the potential success story of PCSK9 inhibitors has been blunted by barriers to patient access. With a sticker price of approximately $14,000 per year per patient, payers and pharmacy benefit managers have imposed strict utilization-management processes, including pre-authorization paperwork and high copays.43 In one analysis, half of adults prescribed PCSK9 inhibitor therapy in the first year of availability never received insurance approval, and one-third who did were unable to fill their prescription due to high copays.44 As a result, only a fraction of potentially eligible patients currently receive therapy.
Identifying the actual production cost of PCSK9 inhibitors is difficult, given that significant research and development investments were required prior to FDA approval of therapy. Monoclonal antibody therapies do require complex manufacturing processes involving cell culture, harvest and antibody purification, among other steps.45 However, material costs are estimated at approximately $2 per gram of antibody, whereas the average price for commercial monoclonal antibody therapy ranges from $2,000 to $22,000 per gram, depending on the antibody.46,47,48 Calculating the actual cost to the pharmaceutical company per gram of antibody is difficult, but a balance between market demand and development cost is factored into the retail price. Therefore, naming a “fair price“ is difficult, but in the case of PCSK9 monoclonal inhibitor therapy, the lack of insurance coverage for the current price is affecting utilization by patients. Although PCSK9 inhibitor therapy has been proved to reduce ASCVD risk in patients who receive it, its broad clinical importance is mitigated by patients’ lack of access. Future discovery programs would benefit not only from consideration of the scientific potential of therapy, but also from planning for patient access and utilization hurdles.
Key to Success: Academic-Industry Partnership
The scientific story of PCSK9 highlights how the strengths of both academic science and the pharmaceutical industry were leveraged to produce an FDA-approved therapy at an unprecedented pace. Once the importance of PCSK9 was validated through academic discovery, the pharmaceutical industry took the lead in developing potential therapies for PCSK9 inhibition. Partnership between academic medicine and industry continued through the clinical trial process, and in a remarkably short time frame, PCSK9 inhibitors were approved for clinical use.
The PCSK9 inhibitor academic-industry partnership highlights the potential benefit of a collaborative discovery paradigm, given the contemporary financial landscape for industrial drug development. The current cost to successfully reach a new drug approval exceeds $2.6 billion, with the included cost of failed compounds estimated in the price tag.49,50 The lengthy timeline and exorbitant cost of getting a new therapy approved has forced pharmaceutical companies to adapt. The mega-mergers between pharmaceutical companies in 1995-2005 resulted in large corporations with broad research and development interests across multiple clinical specialties and therapeutic modalities.51,52 By contrast, the last 10 years have seen a shift in the pharmaceutical industry to leaner companies with more focused interests in specific arenas of therapy.53
The financial risk of drug development is not trivial for industry, and the potential for academic partnership to mitigate some of the risk has sparked change. Currently, only 11.3% of investigational drugs reaching clinical trials receive FDA approval.54,55,56 It is crucial that pharmaceutical companies identify drugs with significant potential for patient therapeutic benefit. This process requires a substantial pre-clinical research commitment, typically spanning biochemistry, molecular biology and model system testing (often in both cellular and animal models). Partnering with academic researchers allows pharmaceutical companies to benefit from the laboratory expertise of groups already well-versed in techniques and pertinent biological pathways. This approach has become more important as therapies have expanded beyond chemical compounds and into biologic treatments, such as immunotherapies. By allowing much of the preclinical discovery to occur in academic laboratories, pharmaceutical companies can identify targets with the best chance of therapeutic impact in clinical trials. This model of development has become more frequent in recent years; between 2000 and 2004, 79% of biologic therapies cited academic research as part of their therapy development. By 2014, the proportion of academic involvement in biologic therapy patent applications rose to 100%.57 Multiple challenges in translational research remain. Funding for research continues to be difficult for both academic and pharmaceutical companies; NIH funding of academic research continues to decline, and expiring patents put pressure on industry revenue.58,59 With federal grant pay lines at historical lows,60 academic laboratories have needed to search for alternative funding sources. Collaborating with pharmaceutical companies can fund significant basic science research through several mechanisms, including focused seed grants or fee-for-service contracts. Pharmaceutical companies can also partner with a principal investigator and sponsor a research program, similar in scope to traditional grant funding. In certain instances, pharmaceutical companies can sponsor a relationship with multiple researchers or an entire department to fund a large program of research.61 These models of pharmaceutical companies funding academic science allow labs to continue rigorous discovery research in an era of stagnant federal funding opportunities. Partnering with academic programs allows pharmaceutical companies to leverage the strengths inherent to basic science research. Focusing on drug targets with proven biological importance, such as was done with PCSK9, can partially de-risk the drug discovery process.62,63,64,65,66 Academic laboratories are also uniquely positioned to develop and utilize novel discovery platforms ranging from informatics, chemical synthesis and genomics to modeling and physiology. Collaboration allows pharmaceutical companies to benefit from the expertise of academic science and leverage novel targets into potentially more successful therapies.
New Pathways for Translation and Commercialization
Although the PCSK9 timeline exemplifies how quickly therapies can be approved through academic-industry partnership, the translation from discovery to commercialization poses hurdles that are unfamiliar territory for many academic scientists. The landscape of scientific discovery commercialization changed drastically with the passage of the Bayh-Dole Act in 1980, which allowed academic institutions to own patents that resulted from federally funded research. The ability to patent academic discoveries opened the door to the translation of bench science, but navigating the process of commercialization remained a challenge. In 2004, the FDA reported that despite basic biomedical knowledge expanding exponentially, the translational gap between discovery and application was also expanding. The NIH similarly identified the wide gap in translational science, and in 2003, the NIH Roadmap program began focusing on accelerating translation from bench discovery to bedside therapy. Dr. Elias Zerhouni, director of the NIH at the time, highlighted the importance of forming industry collaborations, noting, “The private sector will play an essential role in this new paradigm.“ The NIH formed the National Center for Advancing Translational Sciences, aimed at fostering development and commercialization of translational technologies. Several federally sponsored programs, including Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR), were developed to bridge the translational gap. These programs are aimed at small businesses and research organizations that need assistance with research and development of technology with commercialization potential. In the decade following these initiatives, the number of patents based on academic research increased by 82%. Universities have also recognized the importance of facilitating the commercialization of scientific discoveries. Many academic institutions have dedicated groups to help academic innovators navigate the process of licensing and communication.
Since their groundbreaking discovery of PCSK9 mutations, Drs. Abifadel and Boileau have continued a unique model of collaborative research. Whereas their initial research was supported by government and foundational research grants through Dr. Boileau’s academic laboratory, an international research foundation has allowed them to continue to collaborate, despite Dr. Boileau’s academic research lab being located in Paris and Dr. Abifadel’s academic research being located in Lebanon. Inserm (Institut National de la Santé et de la Recherche Médicale), a public scientific and technological institute under the French Ministries of Health and Research, has allowed for continued collaboration between the labs, and their work together has yielded multiple important contributions to the understanding of the genetics of familial hypercholesterolemia.
One of the main aims of Inserm is the translation of scientific discovery into marketable innovations. To facilitate translation, Inserm contains a division, Inserm Transfert, whose mission is to support the transfer of novel discoveries into industrial partnerships. Inserm Transfert serves to navigate the necessary licensing, guidance and legal barriers to discovery translation. By focusing on translating basic science discoveries into marketable technologies, Inserm has not only streamlined the process of academic-industrial partnership, but also profited from the scientific discoveries made by the institute — with €32 million made from industrial collaborations in 2016 alone. To date, Inserm collaborations have produced more than 13,200 scientific publications and more than 1,650 patents. Inserm is funded both by government (69%) and external resources (31%), such as industry partnerships. These funds are then reinvested into research personnel and projects, producing continued scientific discoveries.
This model of academic-industrial partnership serves as one example of streamlining the process of discovery translation from lab to patient. Inserm’s unique collaborative model has allowed for continued funding of important scientific discoveries, and its track record of patents and publications speaks to how academic-industry collaboration can be productive for all parties involved.
Conclusions
Millions of patients in the U.S. suffer from atherosclerotic cardiovascular disease, and many more are at a high risk of developing ASCVD. With traditional research paradigms, developing statin pharmacotherapy for treatment of ASCVD took more than 75 years. By contrast, using a model of academic-industry partnership, going from discovery to FDA approval of PCSK9 inhibitor therapy for ASCVD took only 12 years (Figure 1).
The success of PCSK9 therapies highlights transformative mechanisms for rapid and effective drug development. Brilliant academic scientists used genetic research in large population- based patient cohorts to identify novel genes. Multiple collaborative academic teams, funded through government programs, foundation support and industry partnerships, expanded upon basic science discoveries and identified therapeutic targets. Several pharmaceutical companies, including Sanofi-Aventis/Regeneron and Amgen, saw the potential in PCSK9 inhibitors and took the lead in developing monoclonal antibodies that were FDA-approved in 2015. The handoff of path-breaking academic discovery to industry development, facilitated by risk capital providers, allowed for an accelerated therapeutic timeline; in less than 12 years from its discovery, PCSK9 moved from a novel gene to a target for commercial immunotherapies.
The accelerated timeline of PCSK9 therapy development resulted from the transfer of information from academics to industry. By utilizing the strengths of academic research for discovery, proof of physiologic relevance and early animal studies, industry was able to take the therapeutic target and develop a biologic treatment with clinical efficacy. Industry benefited from a scientifically validated target, and academics propelled a novel discovery into a therapeutic treatment. This example of academic-industry partnership can serve as a model for future collaboration and rapid translation of basic science discoveries into clinical therapies.
Endnotes
- E.J. Benjamin et al., “Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association,“ Circulation 137, no. 12 (2018): e67-e492.
- R. Chou et al., “Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force,“ JAMA 316, no. 19 (2016): 2008-2024.
- A.M. Navar et al., “Temporal Changes in the Association Between Modifiable Risk Factors and Coronary Heart Disease Incidence,“ JAMA 316, no. 19 (2016): 2041-2043.
- N.D. Wong et al., “Residual Dyslipidemia According to Low-Density Lipoprotein Cholesterol, Non-High-Density Lipoprotein Cholesterol, and Apolipoprotein B Among Statin-Treated US Adults: National Health and Nutrition Examination Survey 2009-2010,“Journal of Clinical Lipidology 9, no. 4 (2015): 525-532.
- J.L. Goldstein and M.S. Brown, “A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins,“ Cell 161, no. 1 (2015): 161-172.
- Ibid.
- J.L. Goldstein, S.E. Dana and M.S. Brown, “Esterification of Low-Density Lipoprotein Cholesterol in Human Fibroblasts and Its Absence in Homozygous Familial Hypercholesterolemia,“ Proceedings of the National Academy of Sciences of the United States of America 71, no. 11 (1974): 4288-4292.
- M. Krieger, J.L. Goldstein and M.S. Brown, “Receptor-Mediated Uptake of Low-Density Lipoprotein Reconstituted With 25-Hydroxycholesteryl Oleate Suppresses 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase and Inhibits Growth of Human Fibroblasts, “ Proceedings of the National Academy of Sciences of the United States of America 75, no. 10 (1978): 5252-5056.
- K. Bloch, “The Biological Synthesis of Cholesterol,“ Science 150, no. 3692 (1965): 19-28.
- A. Endo, M. Kuroda and K. Tanzawa, “Competitive Inhibition of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase by ML-236A and ML-236B Fungal Metabolites, Having Hypocholesterolemic Activity,“ FEBS Letters 72, no. 2 (1976): 323-326.
- R. Collins et al., “Interpretation of the Evidence for the Efficacy and Safety of Statin Therapy,“ The Lancet 388, no. 10059 (2016): 2532-2561.
- C. Baigent et al., “Efficacy and Safety of Cholesterol-Lowering Treatment: Prospective Meta-Analysis of Data from 90,056 Participants in 14 Randomized Trials of Statins,“ The Lancet 366, no. 9493 (2005): 1267-1278.
- Practice: Insights from the PALM (Patient and Provider Assessment of Lipid Management) Registry,“ Circulation: Cardiovascular Quality and Outcomes 11, no. 3 (2018): 004249.
- M. Abifadel, “Mutations in PCSK9 Cause Autosomal Dominant Hypercholesterolemia,“ Nature Genetics 34, no. 2 (2003): 154-156.
- J. Cameron et al., “Effect of Mutations in the PCSK9 Gene on the Cell Surface LDL Receptors,“ Human Molecular Genetics 15, no. 9 (2006): 1551-1558.
- K.N. Maxwell and J.L. Breslow, “Adenoviral-Mediated Expression of PCSK9 in Mice Results in a Low-Density Lipoprotein Receptor Knockout Phenotype,“ Proceedings of the National Academy of Sciences of the United States of America 101, no. 18 (2004): 7100-7105.
- S. Rashid et al., “Decreased Plasma Cholesterol and Hypersensitivity to Statins in Mice Lacking PCSK9,“ Proceedings of the National Academy of Sciences of the United States of America 102, vol. 15 (2005): 5374- 5379.
- University of Texas Southwestern Medical Center (2009). https://www.utsouthwestern.edu/edumedia/edufiles/research/center_translational_medicine/dallas_heart_stu dy/dhs-study-overview.pdf
- R.G. Victor et al., “The Dallas Heart Study: A Population-Based Probability Sample for the Multidisciplinary Study of Ethnic Differences in Cardiovascular Health,“ American Journal of Cardiology 93, no. 12 (2004): 1473- 1480.
- J. Cohen et al., “Low LDL Cholesterol in Individuals of African Descent Resulting from Frequent Nonsense Mutations in PCSK9,“ Nature Genetics 37, no. 2 (2005): 161-165.
- J.C. Cohen et al., “Sequence Variations in PCSK9, Low LDL, and Protection Against Coronary Heart Disease,“ New England Journal of Medicine 354, no. 12 (2006): 1264-1272.
- Collaborative Studies Coordinating Center, D.o.B., Gillings School of Global Public Health, University of North Carolina at Chapel Hill. Atherosclerosis Risk in Communities Study Description, 2018. https://www2.cscc.unc.edu/aric/.
- S. Kathiresan et al., “Six New Loci Associated With Blood Low-Density Lipoprotein Cholesterol, High-Density Lipoprotein Cholesterol or Trigylcerides in Humans,“ Nature Genetics 40, no. 2 (2008): 189-197.
- S. Kathiresan, “Myocardial Infarction Genetics: A PCSK9 Missense Variant Associated With a Reduced Risk of Early-Onset Myocardial Infarction,“ New England Journal of Medicine 358, no. 21 (2008): 2299-2300.
- H. Hobbs, email message, March 7, 2018.
- Ibid.
- E.A. Stein et al., “Effect of a Monoclonal Antibody to PCSK9 on LDL Cholesterol,“ New England Journal of Medicine 366, no. 12 (2012): 1108-1118.
- C.S. Dias et al., “Effects of AMG 145 on Low-Density Lipoprotein Cholesterol Levels: Results from 2 Randomized, Double-Blind, Placebo-Controlled, Ascending-Dose Phase 1 Studies in Healthy Volunteers and Hypercholesterolemic Subjects on Statins,“ Journal of the American College of Cardiology 60, no. 19 (2012): 1888-1898.
- B.M. Everett, R.J. Smith and W.R. Hiatt, “Reducing LDL with PCSK9 Inhibitors—The Clinical Benefit of Lipid Drugs,“ New England Journal of Medicine 373, no. 17 (2015): 1588-1591.
- Patent Docs, “Amgen Inc. v. Sanofi (Fed. Cir. 2017),“ 2017. http://www.patentdocs.org/2017/11/amgen-inc-v- sanofi-fed-cir-2017.html
- Ibid.
- J.G. Robinson, “Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events,“ New England Journal of Medicine 274, no. 16 (2015): 1489-1499.
- P.G. Stegg, “Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment with Alirocumab—ODYSSEY OUTCOMES,“ in American College of Cardiology Annual Scientific Session, 2018, Orlando, FL.
- M.S. Sabatine et al., “Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease,“ New England Journal of Medicine 376, no. 18 (2017): 1713-1722.
- P.M. Ridker et al., “Lipid-Reduction Variability and Antidrug-Antibody Formation With Bococizumab,“ New England Journal of Medicine 376, no. 16 (2017): 1517-1526.
- E.P. van Poelgeest et al., “Antisense-Mediated Reduction of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): A First-in-Human Randomized, Placebo-Controlled Trial,“ British Journal of Clinical Pharmacology 80, no. 6 (2015): 1350-1361.
- K.K. Ray et al., “Inclisiran in Patients at High Cardiovascular Risk With Elevated LDL Cholesterol,“ New England Journal of Medicine 376, no. 15 (2017): 1430-1440.
- P. El Khoury et al., “PCSK9 Mutations in Familial Hypercholesterolemia: From a Groundbreaking Discovery to Anti-PCSK9 Therapies,“ Current Atherosclerosis Reports 19, no. 12 (2017): 49.
- T. Mitchell et al., “Pharmacologic Profile of the Adnectin GMS-962476, a Small Protein Biologic Alternative to PCSK9 Antibodies for Low-Density Lipoprotein Lowering,“ Journal of Pharmacology and Experimental Therapeutics 350, no. 2 (2014): 412-424.
- C. Landlinger et al., “The AT04A Vaccine Against Proprotein Convertase Subtilisin/Kexin Type 9 Reduces Total Cholesterol, Vascular Inflammation and Atherosclerosis in APOE*3Leiden.CETP Mice,“ European Heart Journal 38, no. 32 (2017): 2499-2507.
- D.B. Mark and K.A. Schulman, “PCSK9 Inhibitors and the Choice Between Innovation, Efficiency and Affordability,“ JAMA 318, no. 8 (2017): 711-712.
- A.M. Navar et al., “Association of Prior Authorization and Out-of-Pocket Costs With Patient Access to PCSK9 Inhibitor Therapy,“ JAMA Cardiology 2, no. 11 (2017): 1217-1225.
- A.F. Shaughnessy, “Monoclonal Antibodies: Magic Bullets With a Hefty Price Tag,“ BMJ 345 (2012): e8346.
- B. Kelley, “Industrialization of mAb Production Technology: The Bioprocessing Industry at a Crossroads,“ mAbs 1, no. 5 (2009): 443-452.
- C.P. Milne and A. Malins, “Academic-Industry Partnerships for Biopharmaceutical Research & Development: Advancing Medical Science in the U.S.,“ Tufts Center for the Study of Drug Development, 2012.
- A. Gautam and X. Pan, “The Changing Model of Big Pharma: Impact of Key Trends,“ Drug Discovery Today 21, no. 3 (2016): 379-384.
- Ibid.
- Milne and Malins, “Academic-Industry Partnerships.“
- Biotechnology Industry Organization, “Advancing Translational Research for Biomedical Innovation: Measuring Industry-Academic Connections,“ 2015.
- Ibid.
- M.J. Lamberti and K. Goetz, “Profiles of New Approaches to Improving the Efficiency and Performance of Pharmaceutical Drug Development,“ Tufts Center for the Study of Drug Development, 2015.
- Ibid.
- J. Frearson and P. Wyatt, “Drug Discovery in Academia—the Third Way?“ Expert Opinion on Drug Discovery 5, no. 10 (2010): 909-919.
- N.S. Gray, “Drug Discovery Through Industry-Academic Partnership,“ Nature Chemical Biology 2, no. 12 (2006): 649-653.
- Biotechnology Industry Organization, “Advancing Translational Research.“
- Ibid.
- National Institutes of Health, National Center for Advancing Translation Sciences, 2018. https://ncats.nih.gov/
- National Institutes of Health, NCATS Small Business Opportunities, 2018. https://ncats.nih.gov/smallbusiness
- Biotechnology Industry Organization, “Advancing Translational Research.“
- Duke University Office of Licensing & Ventures, Faculty Innovators, 2018. https://olv.duke.edu/faculty- innovators/
- Inserm, Inserm Facts at a Glance, 2018. https://www.inserm.fr/en/about-inserm/inserm-glance
- Inserm, Inserm Value Creation and Transfer of New Discoveries, 2018. https://www.inserm.fr/en/research- inserm/value-creation-and-transfer-new-discoveries
- Ibid.
- Ibid.
- Ibid.
- Inserm, Inserm Facts at a Glance.
-
-
Driving Innovation
-
Innovations in Cardiovascular Health
-
The Role of Physicians in Driving Innovation
-
The Role of Patient Groups in Driving Innovation
-
Clinical Innovations in Cardiovascular Health
-
What Drives Innovation in CV Health?
-
The Rise of Academic and Contract Research Orgs
-
Federal Regulations as Accelerators
-
Reimbursement Models
-
Consumer Technology
-
Training Cross-Disciplinary Innovators
-
Conclusion