The Role of Patient Groups in Driving Innovation in Cardiovascular Disease Research and Therapeutic Development
Yumin Gao, B.S.
Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health
Introduction
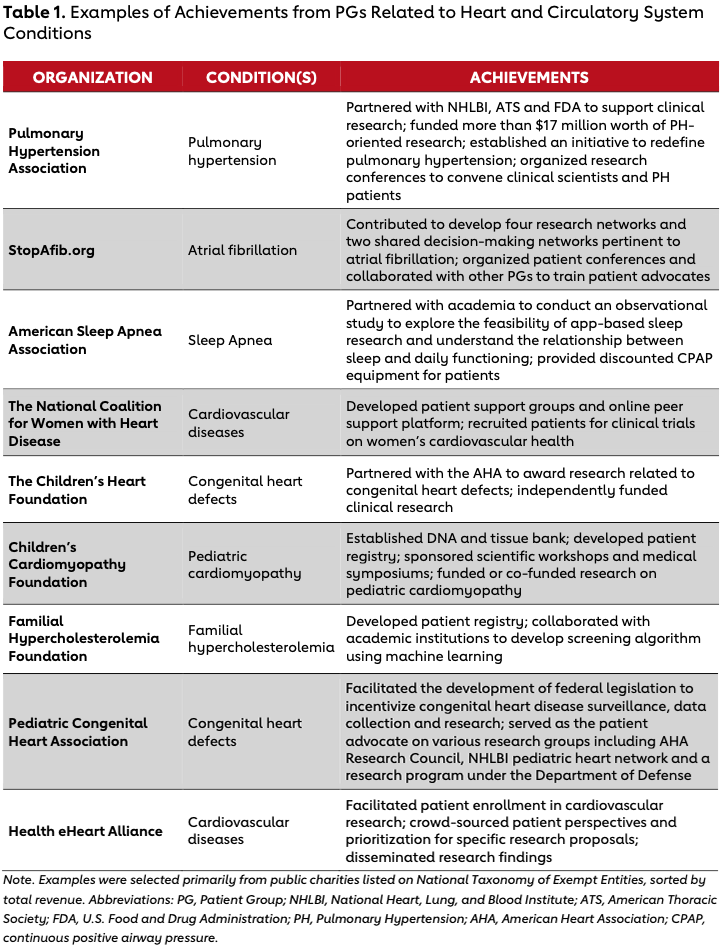
Patient group (PG) is an umbrella term used to encompass patient advocacy organizations, disease-advocacy organizations, voluntary health agencies, nonprofit research foundations and public health organizations.1 Many PGs are founded primarily to represent patients with a particular medical condition, with a goal of raising public awareness and facilitating the development of new diagnostic and therapeutic options for those patients. For decades, PGs have been influential stakeholders in the ecosystem of cardiovascular health care, contributing to medical developments and catalyzing changes in health policy.2,3 Table 1 outlines examples of PGs in cardiovascular disease and their major achievements in driving innovation.
However, the landscape of PGs in cardiovascular disease and how they are driving innovation are changing over time. This article, which draws from both the published literature and from interviews with key leaders and stakeholders in the field, will explore the evolving ways in which PGs promote new discoveries and improved care for patients with cardiovascular diseases. Specifically, we will outline how PGs previously focused more on rare-disease research and advocacy, and on driving therapeutic innovation through traditional research programs. Over time, these groups have expanded their focus to include advocacy for more prevalent cardiovascular diseases, and on influencing the way in which research is being conducted, not just on the products of the research. We argue that in order for PGs to continue driving innovation in cardiovascular diseases, they will need to address certain barriers, particularly around funding transparency and conflicts of interest.
The History of PGs in Cardiovascular Disease: Driving Rare-Disease Research and Therapeutic Development
Traditionally, PGs have voiced the interests of and promoted therapeutic development for patients with rare diseases. This effort took a substantial leap forward in 1983, when a coalition of several rare-disease PGs facilitated passage of the Orphan Drug Act. This legislation reduced regulatory barriers and created economic incentives to develop therapies for rare diseases. For example, the Orphan Drug Act established tax credits, market exclusivity, clinical-research subsidies and fast-track approvals for orphan drugs, which led to a significant increase in the approvals of orphan drugs. Before 1983, fewer than 10 treatments for rare diseases had been approved, but as of 2016, 449 drugs with an orphan indication have been approved.4,5 Furthermore, more than 50% of the new drugs approved by the FDA since 1983 were first-in-class drugs, demonstrating the innovative nature of orphan-drug development since the legislation was passed.6
Besides health-policy advocacy, one primary way in which PGs have catalyzed rare-disease drug development is through research-award programs. The National Organization for Rare Disorders (NORD), an umbrella organization of patient advocacy organizations for rare diseases, has developed an effective research program to promote therapeutic developments. According to Vanessa Boulanger, the director of research programs at NORD, the program is primarily sponsored by patients themselves, corporate matching programs and industry sponsors. Once a certain fund reaches the threshold for a request for proposals, NORD announces the program publicly and reaches out to relevant researchers in the field. As of 2018, this program has resulted in two FDA-approved therapeutics for rare diseases: VEPTR (Vertical Expandable Prosthetic Titanium Rib), an implantable device to treat children with thoracic insufficiency syndrome, and Northera (droxidopa), a medication developed to treat neurogenic orthostatic hypotension.
In addition, NORD hosts a registry platform that collects longitudinal data on patients with various rare diseases, and helps to conduct post-marketing surveillance for rare-disease therapeutics and devices. These registries have been used to speed the process of rare-disease drug development by allowing pharmaceutical companies to quickly evaluate their therapies in real-world settings, and to incorporate patient perspectives into their drug development.
Within the cardiovascular disease space, the Pulmonary Hypertension Association (PHA) is a PG that has grown extensively since its initial foundation in 1992 as an organization that advocates for patients with a rare disease. Pulmonary hypertension is a rare disorder in which the vessels in the lungs have elevated blood pressure, which can lead to heart failure and significant morbidity and mortality; the prevalence is approximately 10 to 52 cases per million.7 PHA drives innovation in the field by supporting five research programs that fund preliminary and proof-of- concept research, particularly among researchers early in their careers. The goal of these programs is to promote transformational research in pulmonary hypertension and to enable young investigators to transition to high-impact awards. A recent internal review conducted by PHA found that its research programs led to at least $30 million in subsequent funding from the National Institute of Health to physician scientists.
Thus, one way PGs in cardiovascular diseases first established themselves as key players in the health care-innovation ecosystem was by focusing on rare-disease advocacy and research through traditional funding pathways.
The Future of PGs in Cardiovascular Disease: Prevalent Diseases and Patient-Centered Research
Over the last decade, the landscape of PGs has evolved such that organizations in the cardiovascular arena are focusing on more prevalent diseases and are increasingly invested in promoting patient-centered research. A prime example of this shift is the Familial Hyperlipidemia (FH) Foundation. The FH Foundation is a nonprofit organization dedicated to improving the diagnosis and treatment of familial hypercholesterolemia (FH) through advocacy, education and research. FH is a genetic disorder of cholesterol metabolism, characterized by a substantial elevation of low-density lipoprotein cholesterol (LDL-C) unrelated to diet or lifestyle. Patients with FH are at a four- to 10-fold increased risk of acute myocardial infarction and coronary heart disease compared with the general population.8 Two types of FH exist: homozygous and heterozygous. Homozygous FH is very rare with an estimated prevalence of 1 in 300,0009; heterozygous FH is more common, affecting about 1 in 250 individuals, or more that 1 million individuals in the U.S.10
The FH Foundation was founded in 2011 and began its mission with a focus on homozygous FH. Its website has sections specific for patients with homozygous FH, informing them of pertinent risk factors, common symptoms and locations of lipoprotein apheresis sites. The foundation also organized patients with homozygous FH to advocate for PCSK9 inhibitors at an FDA Endocrinological and Metabolic Drugs Advisory Committee hearing.11
Over time, the FH Foundation has shifted more efforts to the more prevalent heterozygous form of the disease, and to the importance of a patient-centered approach to research. Though it is more common, heterozygous FH is still under-diagnosed and suboptimally treated, with fewer than 1% of patients in the U.S. receiving an appropriate diagnosis.12 The FH Foundation has developed a national registry to combat this issue. The CASCADE FH Registry captures longitudinal data on the characteristics of FH, adherence of medical therapies and clinical outcomes from patients with FH (mostly those with heterozygous FH) and their caregivers. Information from this patient-driven registry is being used to better understand the course of the disease, its prevalence in the U.S. and current management patterns.
StopAfib.org is another patient-centered organization that empowers a patient community through education and engagement. Atrial fibrillation (AF) is a prevalent cardiovascular disease that increases the risk for thromboembolism and is characterized by an irregular heartbeat in the upper chambers of the heart. Its prevalence was estimated to range between 2.7 million and 6.1 million in the U.S. as of 2014.13 Several key members from StopAfib.org initially served as patient representatives on FDA advisory panels for cardiovascular and renal drugs as well as circulatory-system devices. Recently, StopAfib.org facilitated a summit between patients with AF and clinical researchers in which patients provided their perspectives on clinical studies and how such studies address outcomes that patients are concerned about. As a result, the I-STOP- AFib study was conceived, with an objective of examining the effectiveness of using either N-of-1 trials or symptom surveillance alone to improve AF-related health outcomes and the quality of life for patients.14 The key innovation is that this shifts control of the research from traditional researchers alone to patients, who decide for themselves whether, when and how to share their data. As patient centricity becomes a common theme for clinical research in academia and pharmaceutical industries, patient advocates who are articulate in communicating their perspectives with clinicians, researchers and policy influencers are exceedingly needed. As Mellanie Hills, the founder and CEO of StopAfib.org, points out, a tremendous challenge for her organization is to educate more patients to be effective research advocates who can craft their messages in a way that health professionals and other stakeholders clearly understand.
In an effort to put patients in control of their own data, a research team from the University of California, San Francisco has recently collaborated with StopAfib.org and three other PGs to develop a patient-powered research network (PPRN) named Health eHeart Alliance for the Health eHeart Study, which aims to “gather more data about heart health from more people than any research study has done before.“15 The study enrolls participants and gathers data remotely using mobile technology. The Health eHeart Alliance powers the study by engaging patient principle investigators and a patient advisory board in the research design, patient enrollment, outcome measure, and results dissemination. As of 2016, over 75,000 patients had registered for the Health eHeart Alliance.16 The concept of PPRNs was formalized in 2012 during a national workshop convened by the Patient-Centered Outcomes Research Institute to conduct observational and randomized-outcomes research more efficiently. 17 PPRNs originate in communities of motivated patients with a particular condition or set of conditions, and provide an avenue for these patients to contribute to knowledge generation for patients with the same condition(s).18 Specifically, PPRN members contribute to self-reported and electronic health- record databases and help govern the network’s research activities.
Other PGs such as the Pediatric Congenital Heart Association (PCHA) and American Sleep Apnea Association (ASAA) are also driving patient-centered research by using patients to shape the research agenda. Specifically, PCHA partners with the Children’s Heart Foundation to train patient advocates to voice the research needs of congenital heart disease patients to the U.S. Congress, NIH and Centers for Disease Control and Prevention. The PCHA also insists that all projects it funds or collaborates on must include patients in the study design and conduct processes.
The ASAA is another PG that actively engages in clinical research pertinent to cardiovascular health. One common type of sleep apnea is obstructive sleep apnea, which is associated with increased incidence of hypertension, congestive heart failure, stroke and abnormal glucose metabolism.19 The estimated prevalence of obstructive sleep apnea in developed countries ranges from 3% to 7%.20 To better understand the association between sleep quality and daily functioning among young adults in the U.S., the ASAA is partnering with an academic center to conduct an observational research study based on mobile apps. One major advantage of the ASAA serving as the primary study sponsor is that its relationship with the patient constituents can drive greater participation in research, especially when patient enrollment relies significantly on mobile technology. Moreover, according to Adam Amdur, the chief patient officer at the ASAA, and Dr. Carl Stepnowsky, the principal investigator of the app-based sleep study, the vision of the research partnership goes beyond the collective effort to conduct patient-centered research and seeks to change the traditional roles of PGs, researchers and sponsors such that PGs can fund and lead clinical studies that properly align with the problems patients are interested in, while researchers are playing a consulting role throughout various stages of the research process.
Next Steps: Increasing Funding Transparency and Regulating Conflicts of Interest
As the sphere of influence of PGs continues to increase in the cardiovascular space, an issue has come to light that, if not appropriately addressed, will threaten the ability of PGs to continue driving innovation. The central tenet of PGs is that they represent, first and foremost, the interests of patients. However, funding from outside sources, such as the pharmaceutical industry, could represent a potential conflict of interest. Institutional conflicts of interest are defined as situations in which an institution’s primary interests and missions are jeopardized due to its own financial interests or the interests of its administrative officials.21,22 Such conflicts of interest, real or perceived, could harm constituents of PGs, either by eroding their influence or by leading to biased advocacy for therapeutics that have known risks or minimal benefits.23
Recent studies have shown that pharmaceutical industry funding for PGs is actually quite common, though disclosure of such funding is not. In a recent survey of 104 large U.S.-based patient advocacy organizations, published in the New England Journal of Medicine, 83% reported receiving financial support from industry, though the majority of these did not publish the exact amounts of industry donations.24 Most of the patient organizations that did not report funding from industry did not provide any donor information. Only one of the 104 organizations stated explicitly that it does not receive industry support. In addition, 36% of the organizations reported at least one current industry executive on the governing board. Another national survey of patient advocacy organizations reported that 165 of 245 surveyed organizations (67.3%) endorsed receiving industry funding, and 19 of 160 organizations (11.9%) endorsed receiving more than half of their funding from industry.25
Despite the high prevalence of pharmaceutical financial support, regulation around disclosure policies for PGs is limited. In the above study of 104 patient advocacy organizations, only 12 (11.5%) had a policy that specifically addressed institutional conflicts of interest.26 An examination of the internal grant registry of Eli Lilly and Company in 2011 revealed that only 10% of health advocacy organizations listed on the registry acknowledged Eli Lilly as a grant sponsor, and only 25% of them disclosed the grant from Eli Lilly on their websites.27 This lack of regulation around conflicts of interest is in stark contrast to the disclosure policies that are mandated for individual health care providers.28,29 The Physician Payments Sunshine Act was passed in 2013 to require pharmaceutical companies to publicly report payments and other transfers of value to physicians and teaching hospitals, thus improving the transparency between health care providers and the pharmaceutical industry.30 Although no existing legislation requires individual PGs to adopt comprehensive conflict-of-interest policies, two national patient organizations have developed their own standards of financial disclosure, aiming for more transparent operations. The National Health Council mandates that its member PGs develop a gift-acceptance policy that must include mission-related benefits, types of acceptable donations, types of acceptable donors, specific criteria for refusal and procedures for evaluating gifts.31 In addition, Genetic Alliance, an international organization coalition that focuses on patients with genetic conditions, publicly discloses its funding sources and operational spending on its annual report.
To understand the issue of funding transparency among smaller PGs, we interviewed Bray Patrick-Lake, a patient-engagement expert who founded the PFO (Patent Foramen Ovale) Research Foundation to promote patient-centered clinical research and to increase patient access to unbiased scientific information. The subsequent discussion is based on this interview.
External funding is a critical component for small, volunteer-led PGs to execute scientific agendas and convene multi-stakeholder audiences. The PFO Research Foundation’s 2010 Scientific Summit was funded primarily through unrestricted educational grants in the amount of $5,000 by industrial sponsors since the organization’s annual operating expenses were nominal, as were individual patient contributions. Ultimately, the foundation ceased brick-and- mortar operations and transitioned to an online community group after the FDA considered its industrial sponsorship to be a conflict of interest when rostering the Circulatory System Devices Panel of the Medical Devices Advisory Committee on May 24, 2016. According to Patrick-Lake, the industry sponsors had no board seats or influence over scientific agendas and the grant amounts should have been in accordance with FDA policies, yet the FDA perceived the industry sponsorship to introduce a conflict. Patrick-Lake notes that the organization’s Form 990 could easily be misconstrued without a deeper look since it would show the foundation’s industry funding greatly outweighed that of individual donors that year. Although the regulatory environment for funding transparency is complex and involves multiple stakeholders, an external audit conducted by an independent entity may help small PGs and regulatory agents reach a comfort level where financial sustainability, transparency and ethical obligations are met.
For PGs to maintain the trust of all stakeholders in the health innovation ecosystem, they will need to address concerns around conflicts of interest and improve current reporting and funding policies. Several recommendations on how PGs can improve conflict-of-interest policies and promote their trustworthiness are summarized in Table 2.32,33,34 First, PGs should accurately document and disclose each donation received from a pharmaceutical company, including amounts in exact numbers or reasonable, closed ranges; the name of sponsors; and detailed use of funds. Second, PGs should develop policies to clearly define conditions under which funds from industrial sponsors are acceptable. Ensuring the alignment between the mission of the PG and objectives of industry-sponsored initiatives will help PGs operate in a trustworthy manner. Third, PGs should systematically assess their funding relationships and compliance with conflict-of-interest policies. The Clinical Trials Transformation Initiative, a public-private partnership established to improve the quality and efficiency of clinical trials, recommends that PGs use public charity evaluators such as GuideStar and regulations published by federal institutions to manage internal and external conflicts of interest.35 For PGs that are relatively small and have limited resources for such evaluation, an independent committee can be established to collectively create disclosure policy protocols and evaluate effectiveness of collaborations with pharmaceutical companies. Alternatively, large PGs can catalyze greater transparency by developing standardized disclosure practices and conflict-of-interest policies that can be adopted broadly. Lastly, having multiple financial partners and setting an annual limit for industry funding can boost the sustainability of PGs while allowing them to maintain their autonomy.
Conclusion
Over the last several decades, PGs have been important drivers of research and therapeutic innovation in the cardiovascular space. By broadening their scope to more prevalent diseases and to how research is conducted, these organizations have expanded the ways in which patients can influence the health care ecosystem. To continue to drive change, PGs will need to reassure their constituents and the public that the interests of patients are paramount. Developing and widely instituting conflict-of-interest disclosure policies will go a long way toward ensuring the legacy of PGs in driving cardiovascular innovation.

Endnotes
- Clinical Trials Transformation Initiative, “CTTI Recommendations: Effective Engagement With Patient Groups Around Clinical Trials,“ October 2015.
- D.C. Landy et al., “How Disease Advocacy Organizations Participate in Clinical Research: A Survey of Genetic Organizations,“ Genetics in Medicine 14 (2012): 223-228.
- A.C. Keller and L. Packel, “Going for the Cure: Patient Interest Groups and Health Advocacy in the United States,“ Journal of Health Politics, Policy and Law 39 (2014): 331-367.
- I. Melnikova, “Rare Diseases and Orphan Drugs,“ Nature Reviews Drug Discovery 11 (2012): 267-268.
- National Organization for Rare Disorders, “Orphan Drugs in the United States Providing Context for Use and Cost,“ 2017.
- K.L. Miller and M. Lanthier, “Trends in Orphan New Molecular Entities, 1983-2014: Half Were First in Class, and Rare Cancers Were the Most Frequent Target,“ Health Affairs (Millwood) 35 (2016): 464-470.
- M.M. Hoeper and R.G.J. Simon, “The Changing Landscape of Pulmonary Arterial Hypertension and Implications for Patient Care,“ European Respiratory Review 23 (2014): 450-457.
- L.J. Mundal et al. “Impact of Age on Excess Risk of Coronary Heart Disease in Patients with Familial Hypercholesterolaemia,“ Heart, 2018.
- B. Sjouke et al., “Homozygous Autosomal Dominant Hypercholesterolaemia in the Netherlands: Prevalence, Genotype- Phenotype Relationship and Clinical Outcome,“ European Heart Journal 36 (2015): 560-565
- S.D. de Ferranti et al., “Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES),“ Circulation 133 (2016): 1067-1072.
- M. Brumit, “Representing the HoFH Community at EMDAC,“ June 2015.
- B.G. Nordestgaard et al., “European Atherosclerosis Society Consensus P. Familial Hypercholesterolaemia Is Underdiagnosed and Undertreated in the General Population: Guidance for Clinicians to Prevent Coronary Heart Disease: Consensus Statement of the European Atherosclerosis Society,“ European Heart Journal 34 (2013): 3478-3490a.
- C.T. January et al., “American College of Cardiology/American Heart Association Task Force on Practice G. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society,“ Journal of the American College of Cardiology 64 (2014): e1-76.
- ClinicalTrials.gov, Bethesda (Maryland): National Library of Medicine (U.S.). Individualized Studies of Triggers of Paroxysmal Atrial Fibrillation (I-STOP-AFib); nct03323099. October 2017.
- Health eHeart.
- The National Patient-Centered Clinical Research Network. Heart eheart alliance. 2016
- Patient-Centered Outcomes Research Institute. Pcornet: The national patient- centered clinical research network. March 2018.
- R.L. Fleurence et al., “Patient-Powered Research Networks Aim to Improve Patient Care and Health Research,“ Health Affairs (Millwood) 33 (2014): 1212-1219.
- Ibid.
- N.M. Punjabi, “The Epidemiology of Adult Obstructive Sleep Apnea,“ Proceedings of the American Thoracic Society 5 (2008): 136-143.
- B. Lo and M.J. Field (eds.), “Conflict of Interest in Medical Research, Education and Practice.“ (Washington, D.C.: National Academies Press, 2009.)
- S.L. Rose, “Patient Advocacy Organizations: Institutional Conflicts of Interest, Trust and Trustworthiness,“ The Journal of Law, Medicine & Ethics 41 (2013): 680-687.
- Ibid.
- M.S. McCoy et al., “Conflicts of Interest for Patient-Advocacy Organizations,“ New England Journal of Medicine 376 (2017): 880- 885.
- S.L. Rose et al., “Patient Advocacy Organizations, Industry Funding and Conflicts of Interest,“ JAMA Internal Medicine 177 (2017): 344-350.
- McCoy, “Conflicts of Interest.“
- S.M. Rothman et al., “Health Advocacy Organizations and the Pharmaceutical Industry: An Analysis of Disclosure Practices,“ American Journal of Public Health 101 (2011): 602-609.
- J.S. Ross et al., “Pharmaceutical Company Payments to Physicians: Early Experiences With Disclosure Laws in Vermont and Minnesota,“ JAMA 297 (2007): 1216-1223.
- K.A. Thomas, “Financial Ties Between Doctors and Health Care Firms Are Detailed,“ The New York Times, 2014: B1.
- S. L. Rose, “Patient Advocacy Organizations.“
- National Health Council. Minimum Standards for Acceptance of Voluntary Health Agency Members. January 2017.
- Clinical Trials Transformation Initiative, “CTTI Recommendations.“
- B. Lo and M.J. Field (eds.), “Conflict of Interest in Medical Research.“
- S. Stein et al., “Principles for Interactions With Biopharmaceutical Companies: The Development of Guidelines for Patient Advocacy Organizations in the Field of Rare Diseases,“ Orphanet Journal of Rare Diseases 13 (2018): 18.
- Clinical Trials Transformation Initiative, “CTTI Recommendations. “
- Ibid.
- S.L. Rose, “Patient Advocacy Organizations.“
- Stein et al., “Principles for Interactions.“
-
-
Driving Innovation
-
Innovations in Cardiovascular Health
-
The Role of Physicians in Driving Innovation
-
The Role of Patient Groups in Driving Innovation
-
Clinical Innovations in Cardiovascular Health
-
What Drives Innovation in CV Health?
-
The Rise of Academic and Contract Research Orgs
-
Federal Regulations as Accelerators
-
Reimbursement Models
-
Consumer Technology
-
Training Cross-Disciplinary Innovators
-
Conclusion