The Role of Physicians in Driving Innovation
Angela Lowenstern, M.D.
Division of Cardiology and Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina
Jennifer Rymer, M.D.
Division of Cardiology and Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina
Introduction
Mortality due to cardiovascular disease has fallen drastically in the last two decades,2 at least partially due to notable advances made by individual clinicians who were motivated by gaps they noted in their routine clinical practice. In the field of interventional cardiology, for example, a small pool of entrepreneurial physicians forged the path toward improved devices, education and procedural techniques. In this analysis, we will use the field of interventional cardiology as a prime example in which to examine how cardiovascular innovation can be driven by physicians at the front lines. First, we will discuss several highly innovative interventional cardiologists in the area of device development, with a focus on the primary catalysts and barriers for their success, including an examination of the regulatory landscape and collaborative environment, during the early days of device development. We will then investigate how the funding and regulatory environments have changed over the last two decades, resulting in greater hurdles for interventionalists and other innovative physicians to successfully develop and send to market new devices in an efficient and timely manner. As a result of the current regulatory environment, funding opportunities have become increasingly limited, further hampering device development. We will then examine what new frontiers remain in the field of device development.
Next, we will highlight innovation in the areas of education and technique. Over the last several decades, educational forums and conferences for interventional cardiologists, including Transcatheter Cardiovascular Therapeutics (TCT) and the Society for Cardiovascular Angiography and Interventions (SCAI), have grown out of the efforts of several forward-thinking interventionalists, in order to provide a forum for the interventional community and training opportunities for aspiring interventionalists. Innovations in technique, including the rise of transradial access, have continued to grow, driven by a group of interventional cardiologists who have highlighted the benefits of this approach at educational forums and on social media. In contrast to the barriers innovators in device development currently face, opportunities for innovation in the areas of education and technique remain more easily attainable.
Innovation by Device Development: Solutions for Hurdles in the Catheterization Laboratory
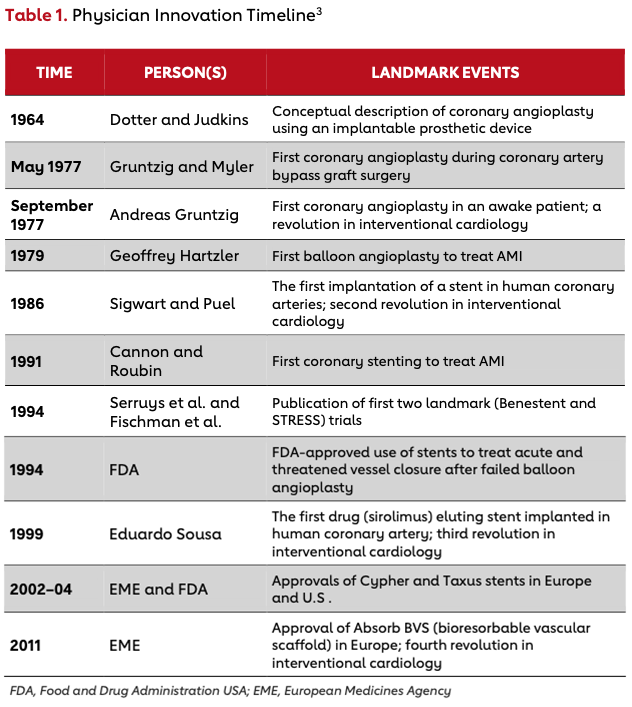
Table 1 illustrates historic milestones in the evolution of interventional cardiology that have taken place over the past six decades.3 Although the interventional community has continued to produce innovative medical devices and techniques, many would say that the 1980s to early 2000s represented the golden age of physician-led innovation in interventional cardiology. This golden age produced early stent technology, vascular closure devices, drug-eluting stents and directional atherectomy. Many of these innovations were rapidly developed in an ecosystem of open collaboration between physicians and industry, and in a different regulatory environment. To explore the ecosystem during the golden age of interventional cardiology, we have interviewed two of the greatest minds and innovators of that time, Dr. John Simpson and Dr. Richard Stack, who drove many of the changes and developments in interventional cardiology. Their experiences allow us to examine the factors present at the time that allowed innovative physicians to excel in interventional cardiology.
John Simpson
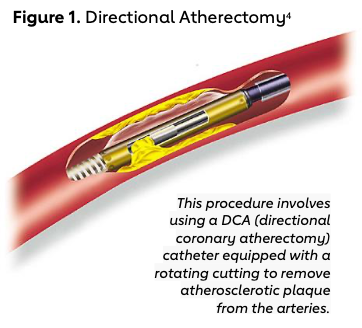
Dr. John Simpson is credited with several important innovations in the field of interventional cardiology, including designing a movable balloon-guidewire system, developing the field of directional atherectomy (Figure 1),4 and developing the Perclose device. A native of West Texas, Simpson aspired to be a veterinarian but did not get into vet school. After working in an immunology lab, he eventually attained admission to Duke for medical school, where he also completed internship and residency. He then pursued a cardiology fellowship at Stanford.
In a recent interview, Simpson described a fortuitous cardiology noon conference, where Andreas Gruentzig was presenting a talk about balloon angioplasty. Given several unfortunate experiences he’d had in the catheterization laboratory where patients’ coronary arteries had been dissected, Simpson wondered if Gruentzig’s balloon could help in the treatment of these dissections. Though Gruentzig shared much about balloon angioplasty with Simpson, he did not share his balloon technology. After traveling back to Stanford, Simpson worked with a plastics company to develop balloon technology over a movable guidewire system, which differed from Gruentzig’s fixed guidewire system. Simpson states that a movable guidewire system was necessary because most interventionalists were not as skilled as Gruentzig.
Simpson also described the environment of collaboration during his early days as a time when device regulation was more relaxed and, in general, collaboration with other innovators was open and free-flowing. He particularly described his relationships with cardiac surgeons, including the famous Norman Shumway, as essential to developing many of the technologies he has over the years. Although Simpson was unquestionably successful, the technologies he developed represented paradigm shifts in the field of interventional cardiology, and thus were met with deep skepticism by many of the reputable figures of the day. He credits perseverance and an incredible commitment to “improve the approximation of the truth“ with his personal success.
Simpson has helped to develop and then sell multiple device companies over the course of his career. He credits the success of these companies with his willingness to hire successful business and legal professionals to help direct most nonclinical decisions. This willingness has freed up his time to focus on developing new ideas and identifying new “pain“ areas in interventional cardiology that require a solution. Although he is always looking for a quick “hit,“ he understands that most breakthroughs in device technology take years to adequately develop and test.
Several key factors are involved in how Simpson successfully developed many of his products during the time of rapid development in interventional cardiology. Like other innovators in the field, Simpson was able to identify processes and device improvements that could improve outcomes when options for treating patients were limited and methods to avoid complications were sparse. Because the regulatory environment was not as rigid during the early years of interventional cardiology, innovators such as Simpson could take new ideas, drawn off the inspiration of other innovators such as Andreas Gruentzig, and translate these into reality over a short period of time.
Additionally, because few solutions existed, interventionalists were able to be more aggressive and creative in their approaches to device development, with relatively fewer consequences for failures from the regulatory agencies. However, although Simpson contends that the regulatory environment is now more stringent and difficult to maneuver, he is optimistic about scientific collaboration. His current collaboration with investigators from multiple institutions and countries continues to be as open as during the early days of his career.
Richard Stack
Perhaps one of the most well-known innovators in the field of interventional cardiology is Dr. Richard Stack. Stack completed medical school and his internal medicine residency training at Wayne State University before transitioning to Duke University Medical Center for his cardiology fellowship. While at Duke, he met and worked closely with his mentor, Dr. Joseph Greenfield, then chair of the division of cardiology.
Stack had initially planned for a career in imaging and echocardiography, but after attending a cardiology grand rounds given by Simpson, became inspired by the possibilities for coronary intervention with the use of guide catheters. With the support of Greenfield, Stack began to work with the Duke engineering school to ask what type of device could aid in opening a blocked or stenosed coronary artery. Initially, inspiration came from other medical specialties that were using stents to open blocked ureters, for example. His work on developing and honing stent technology at Duke relied on collaboration across several departments, including biomedical engineering, surgery and cardiology.
Stack describes an environment of open collaboration, collegiality and less restricted institutional and industry regulation. Ideas were openly shared between collaborators at various institutions, without overwhelming concern for ownership and authorship. Additionally, he was given full support during his fellowship to pursue his entrepreneurial interests in stent development with little restrictions from his fellowship program. Finally, he describes the importance of the era of early interventional device development, when the regulatory environment was much more favorable for early innovators. He has ultimately been credited with developing the technology surrounding the bioresorbable stent, as well as earlier drug- eluting stents.
Further, Stack was a pioneer in establishing the critical role of physicians in biotechnology startup companies that developed and disseminated new technologies. Stack has co-founded multiple companies, including Synergy Life Science Partners, Synecor LLC, BaroSense Inc., InnerPulse and TransEnterix, among others. These companies not only delivered medical innovations, but also provided an avenue through which physicians could innovate while still interacting with both academic and industry partners.
Innovation by Education and Collaboration
Beyond the innovation associated with the development of new devices used to aid in the percutaneous treatment of coronary artery disease, the field of interventional cardiology has also evolved in the way that investigators collaborate and educate one another. This evolution has been driven by motivated physicians. What Stack and Simpson describe as an initially small group of individuals across the country openly sharing new ideas has developed into a large field of cardiology with over 6,000 interventional cardiologists in the U.S. alone.5 To allow this rapidly expanding group of interventionalists to convene to share ideas and present research, Drs. Martin Leon and Gregg Stone, innovators in cardiovascular education, helped establish one of the first national conferences for interventional cardiology, the national Transcatheter Cardiovascular Therapeutics meeting. Started as a conference with just a few hundred attendees in 1988, the TCT meeting has grown to be a yearly conference attracting thousands of attendees. Below, we highlight the contributions of Dr. Stone to examine the importance of physicians driving innovation in cardiovascular education.
Gregg Stone
Within this large cohort of interventional cardiologists, Dr. Gregg Stone is one of the most prominent individuals today, playing a role in numerous interventional cardiology device trials and the growth and development of the TCT meeting. Stone attended Johns Hopkins University for medical school before completing an internship and residency at New York Hospital-Cornell Medical Center. He then trained in general cardiology at Cedars-Sinai Medical Center in Los Angeles and interventional cardiology at St. Luke’s Mid America Heart Institute. After fellowship, he joined a practice in Palo Alto, California, where he first began his career with research collaboration and education.
After about 10 years in California, Stone moved back to the East Coast, where he partnered closely with Dr. Marty Leon in the early stages of the TCT meeting to develop it into the prominent educational and collaborative conference it is today. Initially focusing on coronary interventions alone, the meeting now also includes peripheral vascular disease and structural heart disease — all areas where innovation in device development and technique progression has led to improvements in patient care. However, the meeting’s focus is not only the presentation of new results, but also the opportunity to create a nexus of stakeholders in interventional cardiology from around the world — including investors, analysts, venture capitalists, physician investigators and general cardiologists — to foster collaboration. New innovations and competitions for the best innovation of the year are given center stage, allowing for improved alignment of investors and investigators.
Innovation by Technique: The Next Frontier
Beyond innovation in the development of devices and in how cardiologists collaborate with and educate one another, creative physicians have also moved the field of interventional cardiology forward in terms of procedural technique. The transradial approach for PCI has grown precipitously in Europe, compared with relatively slow growth over the past decade in the U.S. In a recent analysis of PCI trends reported out of the CathPCI Registry from 2011 to 2014, the transradial approach had increased from being used in 10.9% of PCI cases in 2011 to 25.2% of cases in 2014.6 However, this increase represents slow growth, especially with regard to European utilization, and in light of the multiple randomized clinical trials demonstrating improved outcomes in the reduction of access-site bleeding, vascular complications and net adverse clinical outcomes.7-9
During the time of radial PCI expansion, technological developments allowed interventional cardiologists to tackle another challenge in the cardiac catheterization lab, namely, the exposure to ionizing radiation and the requirement to wear heavy lead-shielding aprons. In 2011, Granada et al. showed preliminary safety and feasibility of a robotic angioplasty system, allowing interventional cardiologists to deliver and manipulate guidewires, angioplasty balloons and even stents from a remote-control console.10 This innovation stemmed from experiences in other medical fields, notably, the successful implementation of robotic technology in surgical procedures decades before.11 By 2012, the FDA approved the use of the CorPath 200 System (Figure 2) for PCI, opening a new door in interventional cardiology.
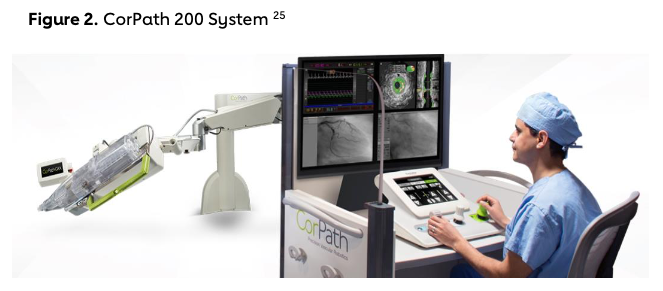
Shortly after FDA approval, results of the PRECISE Study, a multicenter safety and feasibility investigation, were released and showed procedural success in 160 of 164 (97.6%) cases and a 95.2% decrease in the radiation exposure for the primary operator.12 More recently, use of robotically assisted PCI in more complex lesions in the CORA PCI study was shown to be safe and feasible, with a clinical success rate of 99.1%.13 No difference emerged in stent utilization, contrast utilization or patient radiation exposure in patients who underwent robotically assisted PCI compared with the standard manual PCI. Although the full implications of robotically assisted PCI remain to be seen, the successful implementation of robotically assisted PCI has the potential to change the fundamental methods by which PCI procedures are performed.
Regulatory Effect on Physician-Led Innovation
Motivated and entrepreneurial physicians have had a profound impact on interventional cardiology — from how coronary artery disease is treated to the techniques used to perform procedures and the methods of disseminating information. However, parallel with the evolution of devices, collaboration and technique over time, a shift has also occurred in the environment of medicine and the regulation associated with new devices and technique. Simpson and Stack describe an early field that was ripe with opportunity to develop devices in a setting of less restrictive regulation, a topic discussed below. However, as may be expected with any significant change in the treatment of patients, such a revolutionary idea to treat coronary artery disease with percutaneous methods met significant resistance from the medical community. At the time, coronary artery bypass surgery was the treatment of choice, and deviation from it was viewed with skepticism.
In the current cardiovascular treatment environment, treatment of coronary artery disease with percutaneous intervention has become the standard of care for many patients, particularly in the setting of ST-elevation myocardial infarction where door-to-device time is a performance measure that remains a focus of quality-improvement initiatives.14,15 In contrast to the resistance regarding the feasibility and appropriateness of percutaneous intervention that early interventional cardiologists faced, new challenges in innovation largely revolve around regulatory standards and the costs associated with the development, testing and validation of new tools and techniques.
The FDA approval process is constructed around the classification of devices based on risk, with class I devices representing low risk, class II moderate risk and class III high risk. Unsurprisingly, the process for approval of a class I device is less arduous than that for a class III device, which often requires premarket approval (PMA), the most stringent type of device application. As implantable or life-sustaining/support devices, cardiovascular stents, atherectomy catheters and most other cardiovascular intervention devices are categorized under class III and can require lengthy and expensive trials to qualify for PMA, or pre-market notification (PMN) in the case of a predicate device. For example, SPIRIT III, the pivotal trial leading to FDA approval for the Xience V everolimus-eluting coronary stent, enrolled over 1,000 patients at 65 institutions across the U.S. and included clinical follow-up for 12 months.16,17 At the time of the initial development of coronary stents, the FDA had only recently adopted the Medical Device Amendments of 1976, a piece of legislation that laid the foundation for medical-device evaluation by the FDA. Additional revisions and legislation over time have led to the current environment where bringing a device to market can take, on average, three to seven years and cost millions of dollars.18,19 As stated by Dr. Martin Leon, “Progress in [medical] technology usually results less from individual genius and more from collective effort and social, political and economic forces that come together to create an ecosystem which fosters innovation.“ The current regulatory and funding ecosystem can hamper the innovations of many physicians and entrepreneurs.
Although lengthy, the FDA device approval process aims to minimize risk to patients through significant testing and evaluation prior to device approval and marketing. To have their device approved, developers must show the device is not only safe, but also efficacious. Conversely, device approval in the EU requires only that the device demonstrates safety and that it works as intended — proof of efficacy is not required.20 This scenario may lead to substantial differences in the data required for device approval; whereas a device could be approved by the EU based on promising pilot data, the FDA might require additional studies prior to PMA. These contrasting requirements for device approval have led to an estimated lag time of up to seven years for approval in the U.S. behind that in Europe21 and can represent a much more significant upfront cost before device approval and marketing. However, upon device approval in the EU, the device still requires reimbursement approval prior to use, and this process can be substantially longer in the EU compared to the U.S.21 Additionally, early approval, whether in the U.S. or Europe, does not always align with improved patient outcomes. The approved device might not provide any additional benefit over those already widely used in practice and, in some cases, may actually pose higher risk or worse outcomes that are discovered with post- market safety surveillance.
The increased regulatory environment that exists today, compared with the environment that predominated during the days when Simpson and Stack were developing their signature innovations, has also hampered the innovative physician by indirectly decreasing funding for new devices. In a 2012 National Venture Capital Association survey, 42% of health care investors and venture capital firms stated that they were decreasing funding to device companies and entrepreneurs because of the increased time for devices to obtain regulatory approval, and 61% noted the overall regulatory barriers of the FDA had contributed to decreased funding.22 Indeed, in 2007, 116 venture capital firms raised $720 million in device-related funding, which dipped to $200 million in 2011. In addition to the impact of increasing regulatory hurdles, funding barriers are also now a major obstacle for success compared with the relatively free-flowing funding that existed several decades ago.
Beyond the challenges associated with device approval and funding, more recently, device development and marketing faced a new challenge in the medical device excise tax — a new 2.3% tax on the sale price of medical devices, applicable between Jan. 1, 2013, and Dec. 31, 2015. As an excise tax, the percentage is taken from the manufacturer’s revenue, and thus, regardless of whether a company is profitable, the tax remains. A survey completed by the Advanced Medical Technology Association, evaluating the implications of the tax on device development within their member companies, showed job loss, a reduction in research and development, deferred or canceled capital investments, and a reduction in employee compensation after a year of implementation.23 At the end of 2015, a two-year moratorium on the medical device excise tax was signed into law and an additional two-year moratorium just began in January 2018. Although the medical device excise tax remains on hold with the moratorium in place, the ongoing implications of a possible reimplementation in 2020 remain uncertain.
In 2015, the FDA awarded a grant to the Medical Device Innovation Consortium to create the National Evaluation System for health Technology Coordinating Center (NESTcc). NESTcc grew out of the increasing complexities of the regulatory environment for medical devices and the need to increase the responsible use of real-world evidence across the total product life cycle for a diverse set of stakeholders.24 Each year NESTcc selects demonstration projects, ranging from enhancing development of devices to manage peripheral arterial disease to incorporating data collection for clinical trials in a registry-based infrastructure. The purpose of NESTcc is to support innovation in real-world data capture in an efficient and cost-effective manner with the expectation that the knowledge gained from these projects can then be used to support further device-related projects in the future. The overarching goal of NESTcc is to help device-related projects overcome many of the barriers that currently exist.
Conclusion
The field of interventional cardiology has seen tremendous innovation since 1977 when Dr. Gruntzig performed the first angioplasty in an awake patient. What began with significant resistance against a revolutionary way of performing coronary interventions now exists as its own subspecialty in cardiovascular medicine. Over the past five decades, innovation in the field of interventional cardiology has largely been driven by entrepreneurial physicians and interventional cardiologists, such as Richard Stack and John Simpson. Even the very first balloon angioplasty was conducted using a catheter invented by Dr. Fogarty, one of the most innovative physicians of his generation. However, innovation has not been limited just to the device space, but has also expanded into the educational arena and into new techniques and approaches to perform PCI.
During the early days of device development and innovation, collaboration was free-flowing, the environment was wide open to practice improvement, and funding was more widely available. Innovators were largely able to practice in an ecosystem where “thinking outside the box“ and diverging from the status quo were celebrated and rewarded. Now, with an increasingly stringent regulatory environment and the resulting difficulties in obtaining funding for new device development, innovative physicians and entrepreneurs have to seek out significant financial backing for ideas that may not be as radical but are more likely to get approved and funded. Additionally, as we have highlighted, programs such as NESTcc have sprung up out of the need to guide innovators in the device and regulatory spaces.
Beyond the implementation of new devices to percutaneously treat coronary artery disease, the growth of educational forums and techniques used in the cardiac catheterization laboratory have both become areas of innovation. Under the direction of innovators such as Gregg Stone, interventional educational conferences such as TCT and SCAI have helped promote interventional research, training and education for current interventionalists as well as fellows in training. Although the uptake of radial PCI remains low at many institutions and the implementation of robotic-assisted PCI is still sparse, ample opportunities remain for both improvement in PCI technique and the expansion of the transradial approach. Though innovation in the device development space has become increasingly more difficult over the past two decades due to increasing regulatory and funding restrictions, opportunities for innovation in the areas of education, technique and process improvement remain ripe.
Despite the changing regulatory environment and the funding required for the development and approval of new devices, interventional cardiology has continued to make huge advances toward improving the care of patients with atherosclerotic cardiovascular disease. Much of this success can be attributed to the ongoing commitment of leaders in the field to innovation in the areas of device development, education and process improvement. Going forward, continuing to foster growth in each of these areas will be important. We foresee ongoing innovation aimed at device development leading to a more individualized method of coronary artery revascularization — one stemming from the increased utilization of intracoronary imaging techniques and technology to guide procedures based on the unique anatomy of the atherosclerotic plaque. However, innovators in device development will have to continue to adapt to the ongoing challenges of device regulation and funding mechanisms to succeed in this area.
Additionally, we believe that educational opportunities will continue to abound in the growing interventional cardiology conferences available for interventional cardiologists and fellows in training. However, to best utilize these spaces for education and collaboration, an ongoing emphasis on the formation of partnerships across fields — including physicians, industry partners and additional financial backers — will remain essential. In this way, we can not only best support the dissemination of information on devices and techniques in interventional cardiology, but also help foster future innovations.
Finally, moving forward, we envision an ongoing expansion of innovative procedural techniques. Although the transradial approach has thus far struggled in national uptake, we believe that ongoing pressures — from health systems, insurers and patients — will likely lead to increased utilization of this technique that promotes same-day discharge and lower rates of many complications. Overall, if the optimism of some of the current interventional leaders is any indication of the innovations to come, physicians at the front lines will remain a source of ongoing innovation and improvement in the treatment of one of the most prevalent disease processes in the world today.
Endnotes
- G.A. Roth et al., “Global and Regional Patterns in Cardiovascular Mortality From 1990 to 2013,“ Circulation 132 (2015): 1667- 1678.
- GBD 2016 Causes of Death Collaborators, “Global, Regional and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990-2013: A Systematic Analysis for the Global Burden of Disease Study 2013,“ Lancet 385 (2015): 117- 171.
- J. Iqbal, J. Gunn and P.W. Serruys, “Coronary Stents: Historical Development, Current Status and Future Directions,“ British Medical Bulletin 106 (2013): 193-211.
- “Atherectomy (Directional Coronary Atherectomy or DCA),“ Advanced Cardio Services, 2018. https://advancedcardioservices.com/atherect omy-directional-coronary-atherectomy-or- dca/
- T.A. Bass, “Interventional Cardiology U.S. Workforce: Current Challenges,“ Circulation: Cardiovascular Interventions 7 (2014): 733- 735.
- F.A. Masoudi et al., “Trends in U.S. Cardiovascular Care: 2016 Report From 4 ACC National Cardiovascular Data Registries,“ Journal of the American College of Cardiology 69 (2017): 1427-1450.
- E. Romagnoli et al., “Radial versus Femoral Randomized Investigation in ST-Segment Elevation Acute Coronary Syndrome: The RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) Study,“ Journal of the American College of Cardiology 60 (2012): 2481-2489.
- I. Bernat et al., “ST-Segment Elevation Myocardial Infarction Treated by Radial or Femoral Approach in a Multicenter Randomized Clinical Trial: The STEMI-RADIAL Trial,“ Journal of the American College of Cardiology 63 (2014):964-972.
- M. Valgimigli et al., “Radial Versus Femoral Access in Patients With Acute Coronary Syndromes Undergoing Invasive Management: A Randomised Multicentre Trial,“ Lancet 385 (2015): 2465-2476.
- J.F. Granada Jet al., “First-in-Human Evaluation of a Novel Robotic-Assisted Coronary Angioplasty System,“ JACC: Cardiovascular Interventions 4 (2011): 460- 465.
- B.P. Jacob and M. Gagner, “Robotics and General Surgery,“ Surgical Clinics of North America 83 (2003): 1405-1419.
- G. Weisz et al., “Safety and Feasibility of Robotic Percutaneous Coronary Intervention: PRECISE (Percutaneous Robotically Enhanced Coronary Intervention) Study,“ Journal of the American College of Cardiology 61 (2013): 1596-600.
- E. Mahmud et al., “Demonstration of the Safety and Feasibility of Robotically Assisted Percutaneous Coronary Intervention in Complex Coronary Lesions: Results of the CORA-PCI Study (Complex Robotically Assisted Percutaneous Coronary Intervention),“ JACC: Cardiovascular Interventions 10 (2017): 1320-1327.
- P.T. O’Gara et al., “2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines,“ Journal of the American College of Cardiology 61 (2013): e78-140.
- H. Jneid et al., “2017 AHA/ACC Clinical Performance and Quality Measures for Adults With ST-Elevation and Non-ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures,“ Journal of the American College of Cardiology 70 (2017): 2048-2090.
- G.W. Stone et al., “Randomized Comparison of Everolimus-Eluting and Paclitaxel-Eluting Stents: Two-Year Clinical Follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III Trial,“ Circulation 119 (2009): 680-686.
- G.W. Stone et al., “Comparison of an Everolimus-Eluting Stent and a Paclitaxel- Eluting Stent in Patients with Coronary Artery Disease: A Randomized Trial,“ JAMA 299 (2008): 1903-1913.
- G. Van Norman, “Drugs, Devices and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices,“ JACC: Basic to Translational Science 1 (2016): 277-287.
- Ibid., 170-179.
- D.B. Kramer, S. Xu and A.S. Kesselheim, “Regulation of Medical Devices in the United States and European Union,“ New England Journal of Medicine 366 (2012): 848-855.
- G. Van Norman, “Drugs and Devices,“ 399-412.
- J.V. Cadet, “Future of CV Innovation: The World is Flattening,“ Cardiovascular Business (2012).
- “Impact of the Medical Device Excise Tax,“ AdvaMed, 2014. https://www.advamed.org/sites/default/files/ resource/417_AdvaMed_DeviceTaxReport_0117 14_FINAL.pdf
- J.L. Losby et al., “Value of a Facilitated Quality Improvement Initiative on Cardiovascular Disease Risk: Findings from an Evaluation of the Aggressively Treating Global Cardiometabolic Risk Factors to Reduce Cardiovascular Events (AT GOAL),“ Journal of Evaluation in Clinical Practice 21 (2015): 963-970.
- CorPath GRX, 2018. http://www.corindus.com/corpath-grx/how- it-works.
-
-
Driving Innovation
-
Innovations in Cardiovascular Health
-
The Role of Physicians in Driving Innovation
-
The Role of Patient Groups in Driving Innovation
-
Clinical Innovations in Cardiovascular Health
-
What Drives Innovation in CV Health?
-
The Rise of Academic and Contract Research Orgs
-
Federal Regulations as Accelerators
-
Reimbursement Models
-
Consumer Technology
-
Training Cross-Disciplinary Innovators
-
Conclusion